
What is Mitochondria regeneration?
Many diseases are related to mitochondrial damage, which easily leads to cell death. Once cells in many vital organs die, they are difficult to regenerate and repair. Therefore, regenerative medicine offers patients hope for treatment. The most well-known form of regenerative medicine today is stem cell therapy. Numerous studies and clinical trials have confirmed that stem cell therapy can repair damaged tissues and restore function.
Mitochondrial regenerative medicine is a novel regenerative therapy that utilizes high-quality exogenous healthy mitochondria for transplantation/treatment. It is a cell-based regenerative drug that can enter cells, replace damaged and dysfunctional mitochondria, and improve the function of damaged/aging cells. It is a more advanced and sophisticated technology than stem cell therapy.
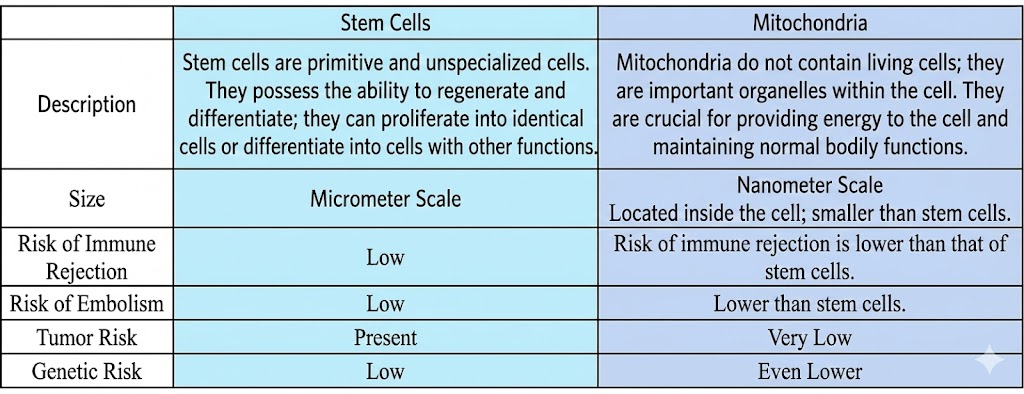
The difference between mitochondria and stem cells
“Mitochondrial regeneration therapy” is a regenerative medicine technology that has received much attention and focus in recent years, marking a new milestone in the application of mitochondrial medicine. Many cutting-edge medical teams have discovered that directly delivering healthy mitochondria to damaged areas, including the heart, brain, and lungs, allows damaged cells to acquire healthy mitochondria, improving damaged tissue and restoring organ function. This can also be considered cell-free therapy; compared to stem cells, mitochondria are much smaller, at the nanometer scale, and carry a lower risk of genetic mutations, vascular embolism, immune rejection, and tumor formation.
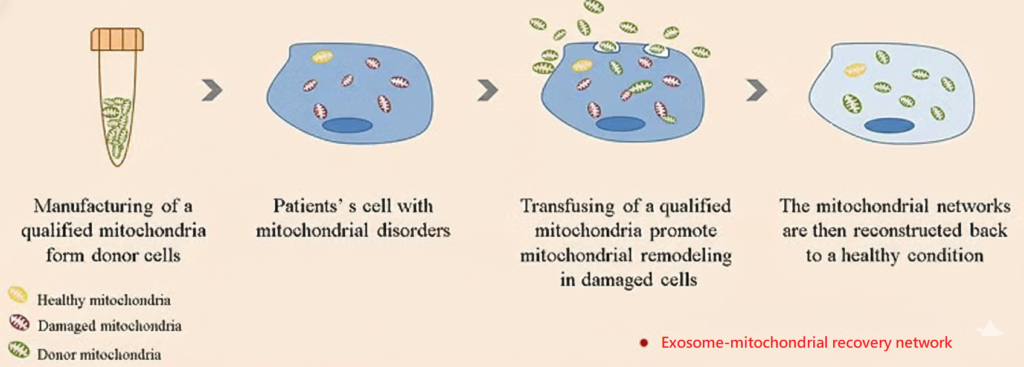
Treatment mechanism
In the damaged area, “healthy exogenous mitochondria” are introduced. Through endocytosis, the mitochondria are engulfed into the cell. At this time, the exogenous mitochondria fuse with the original damaged mitochondria in the cell, repairing the damaged mitochondria. Then, the damaged mitochondria are removed and metabolized through fission, thereby restoring the number and function of mitochondria.
Application of diseases
- Alzheimer’s disease treatment applications
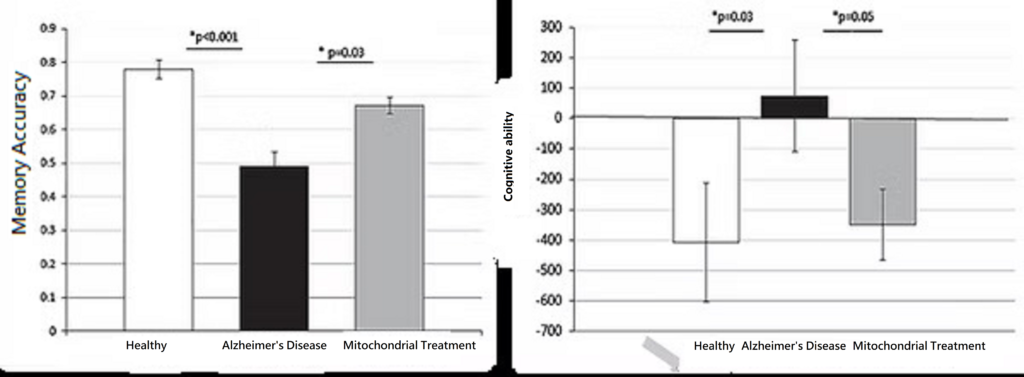
Alzheimer’s disease is a neurodegenerative disease caused by multiple factors. It affects the biochemical reactions of many brain cells, including amyloid beta aggregation, neurofibrillary tangles, oxidative stress, and neuroinflammatory symptoms, ultimately leading to neuronal death. This causes atrophy of the hippocampus, which is responsible for memory, and further triggers lesions throughout the brain. Because neurons are highly dependent on mitochondria to generate energy for survival, damage to mitochondria can lead to the aforementioned lesions.
Because the causes of Alzheimer’s disease are quite complex, current research indicates that there are no effective drugs for treating Alzheimer’s patients. To reduce the limited effectiveness of single-drug treatments for Alzheimer’s, scientists have proposed transplanting active, intact mitochondria as a treatment method. Studies have found that Alzheimer’s mice receiving “mitochondrial reconstruction therapy” showed significantly increased activities of citrate synthase and cytochrome C oxidase, reduced hippocampal neuronal loss, and decreased glial tissue, which also reduced cognitive impairment. Therefore, “mitochondrial reconstruction therapy” offers new hope for treating Alzheimer’s disease.
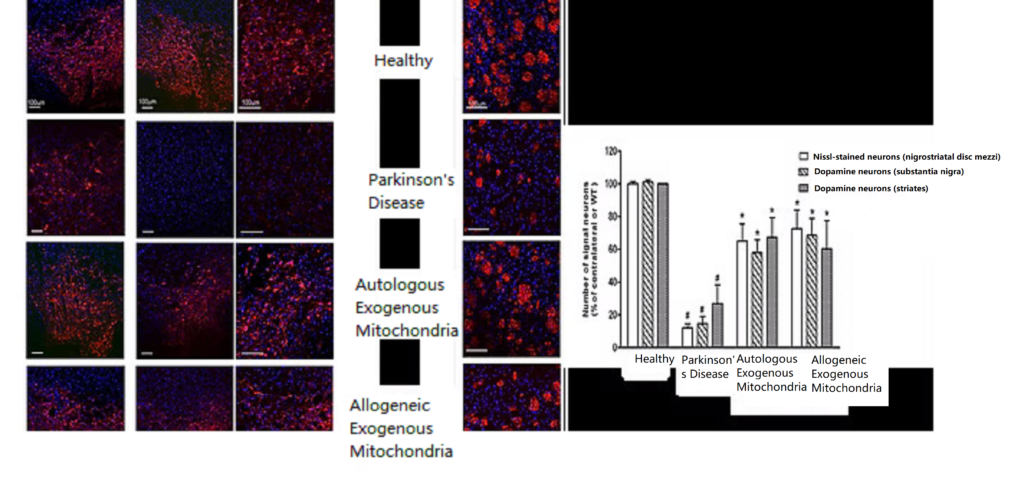
- Parkinson’s disease treatment applications
Parkinson’s disease is caused by the death of dopamine-producing neurons in the substantia nigra of the brain, leading to the inability to produce dopamine and resulting in motor disability. Both genetic and environmental risk factors can cause the death of dopamine neurons, with mitochondria controlling cell fate. In healthy individuals, damaged mitochondria can be metabolized, but when the damage repair mechanism is impaired, mitochondrial damage accumulates, leading to increased oxidative stress and ultimately the inability of mitochondria to produce energy, resulting in the death of dopamine neurons. Current clinical treatments aim to directly or indirectly increase dopamine activity to alleviate motor disability, but they cannot salvage already dead dopamine neurons. Research has found that external mitochondrial therapy in Parkinson’s disease rats restored the number of dopamine neurons in the brain, increased mitochondrial functional proteins, and significantly improved motor function, effectively reducing motor disability caused by Parkinson’s disease. Therefore, “mitochondrial reconstruction therapy” offers a new direction for the treatment of Parkinson’s disease.
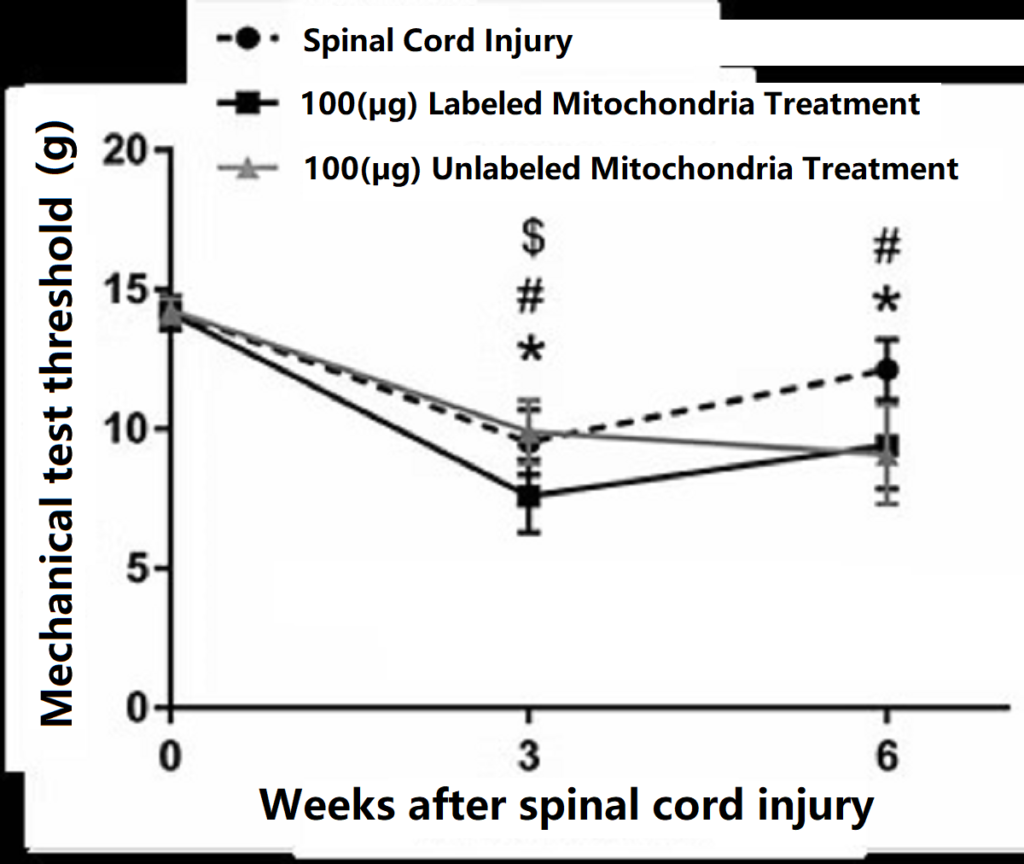
- Applications in the treatment of spinal cord injury
The spinal cord is a peripheral nervous system extending from the brain. Spinal nerves entering and exiting specific segments of the spinal cord control specific parts of the body. The uppermost segment, the cervical spinal cord, controls respiration, neck and upper limb function; the thoracic spinal cord is responsible for the movement of the thoracic and abdominal cavities; the lumbar spinal cord regulates the function of the lower limbs; and the lowest segment, the sacral spinal cord, controls defecation, urination, and sexual function. “Spinal cord injury” refers to acute traumatic injury to the spinal cord and nerves, usually causing varying degrees of damage depending on the specific segment of the spinal cord injured. Injury to the cervical spinal cord can cause quadriplegia; injury to the thoracic, lumbar, or sacral spinal cord can cause lower body paralysis. In addition, spinal cord injury can cause difficulty urinating and defecating, sexual dysfunction, and may also affect breathing and autonomic nervous system function. Because nerve cells do not regenerate easily after death, there is currently no effective treatment for spinal cord injury. Currently, there are many scientific methods aimed at increasing nerve activity and neuronal transplantation. Among them, “mitochondrial reconstruction therapy” has been used to treat spinal cord injuries. Preliminary results have shown that mice treated with mitochondrial therapy showed increased responsiveness to mechanical tests.
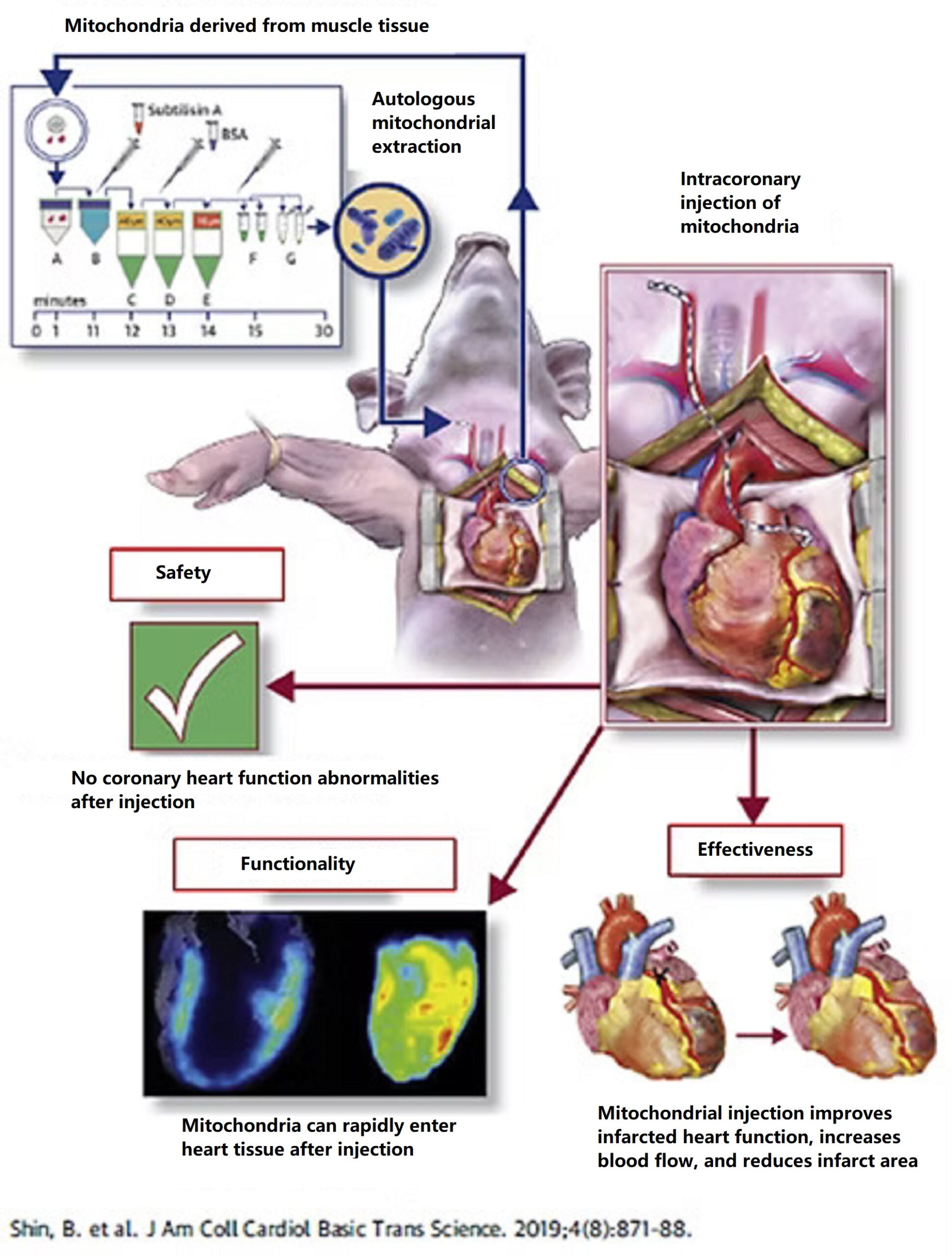
- Application in the treatment of myocardial infarction
When the heart is blocked by blood vessels, blood cannot be transported to the heart tissue, causing myocardial deprivation of oxygen and nutrients. This damage to mitochondria quickly leads to myocardial death. Studies have shown that directly extracting mitochondria from muscle tissue and then injecting them into ischemic myocardium (“mitochondrial reconstruction therapy”) resulted in increased mitochondrial activity and inhibited myocardial cell death in animals receiving this therapy. This effectively improved cardiac contraction and relaxation functions, and the therapy was also safe. In clinical trials, “mitochondrial reconstruction therapy” has also been shown to improve cardiac function in children after myocardial infarction and revascularization.
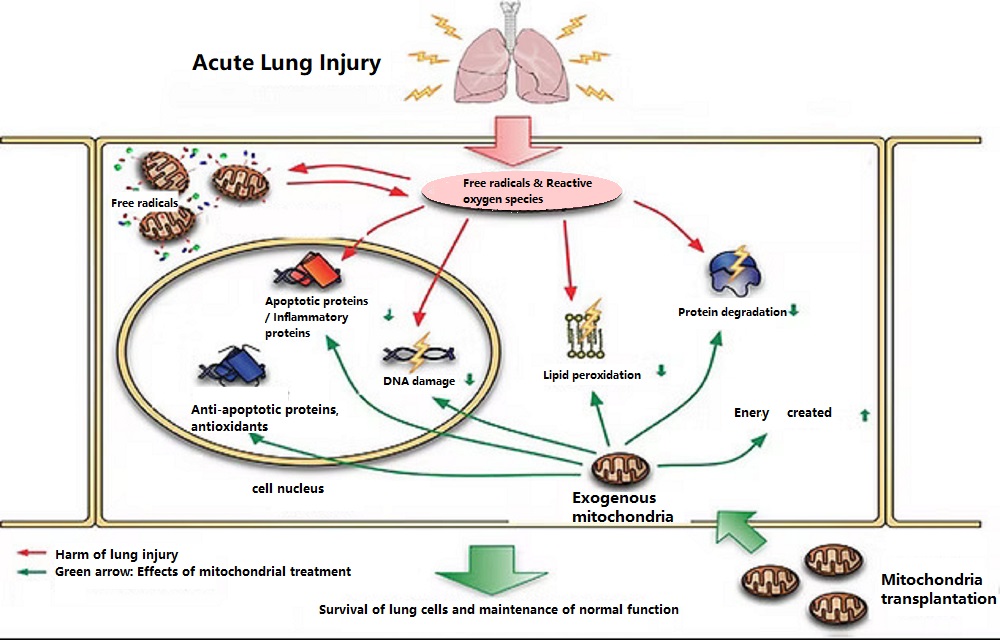
- Mitochondrial therapy for acute lung injury/acute respiratory distress syndrome
Studies have found that in animals simulating lung injury, mitochondrial damage in lung cells is significantly increased, and the damaged lungs further lead to multiple organ failure and death. However, when animals received “mitochondrial reconstruction therapy,” the mitochondrial damage in lung cells was repaired, thus effectively reducing lung cell death. Furthermore, “mitochondrial reconstruction therapy” also reduced immune cell infiltration and the production of inflammatory proteins. Therefore, “mitochondrial reconstruction therapy” can reduce respiratory distress symptoms by maintaining the alveolar epithelial barrier, reducing a cascade of inflammatory responses, and decreasing pulmonary edema, thereby potentially reducing the high mortality rate of acute lung injury.
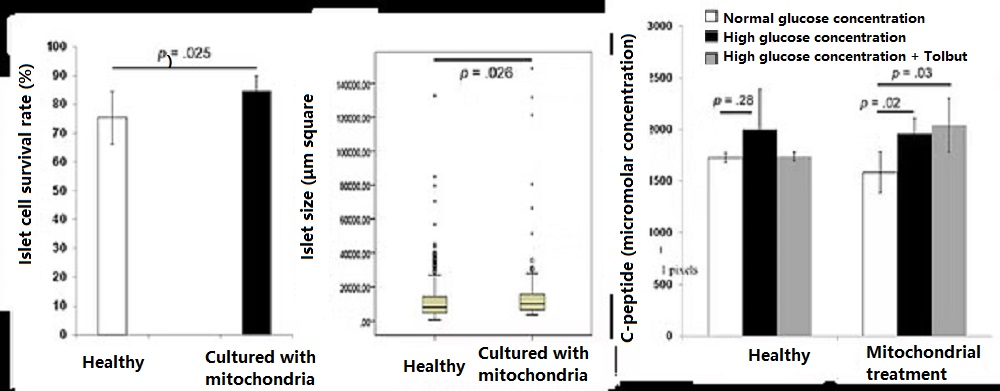
- Applications in the treatment of diabetes
Diabetes has become a global health problem. Type 1 diabetes is a hereditary disease caused by the body’s own immune cells attacking pancreatic tissue, leading to pancreatic cell death. Type 2 diabetes is primarily caused by a high-sugar, high-fat diet, which weakens mitochondria and reduces their function, resulting in significantly increased oxidative stress. Prolonged high blood sugar gradually leads to pancreatic cell death and an inability to produce insulin. Both type 1 and severe type 2 diabetes patients suffer from impaired or dead pancreatic function, preventing insulin production. Preclinical studies have shown that culturing the pancreas and mitochondria together can enhance islet activity and increase insulin release. In the future, “mitochondrial reconstruction therapy” may be clinically applied to treat diabetes, including type 1 and type 2 diabetes, to increase the survival rate of islet cells and insulin secretion.
Learn more about mitochondria

